Introduction
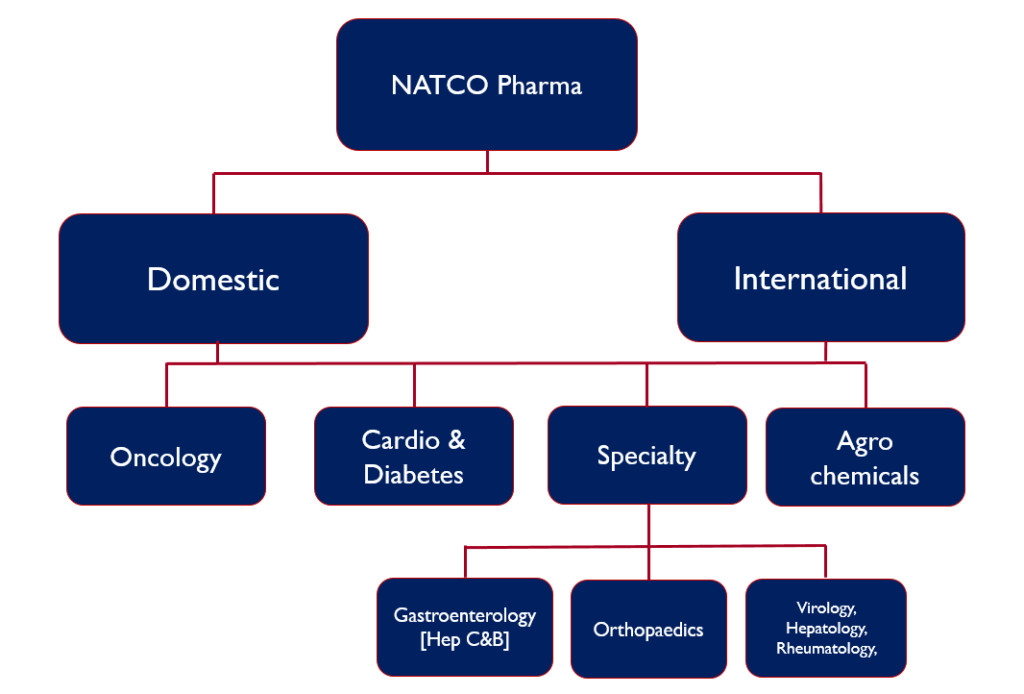
- Natco pharma, is an India based pharmaceutical company involved in the manufacture and marketing of APIs and Finished Dosage Formulations.
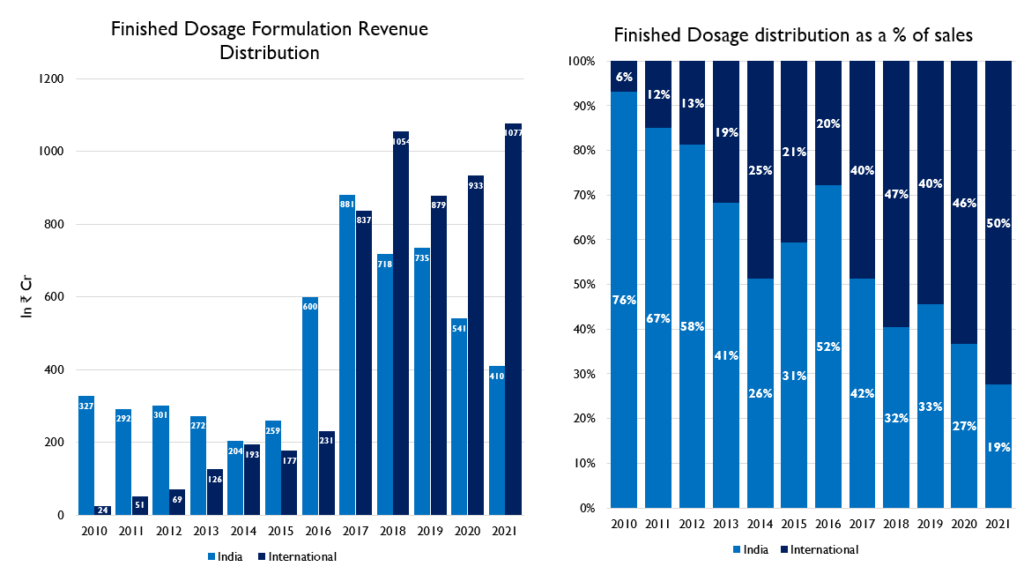
- NATCO has established presence in the Domestic as well as International markets through its subsidiaries in markets like Brazil and Canada. .The US has the largest contribution out of their international business.
- The company is involved in a range of therapeutic areas such as Oncology, Cardio-Diabetic segment and the Specialty segment. Oncology has been their strongest suit and the most stable segment over the period. The therapeutic category has been a very strong business not only in India but in the US markets as well.
- The company’s business model thrives on challenging the patents of the innovator molecules or entering into a settlement with them (para IV filing). The molecules involved in the R&D pipeline are generally complex molecules through which the management believes they can derive the most value from.
Management
Sri G.S Murthy – Independent director and Chairman of Natco Pharma. Mr. Murthy’s qualifications include LL.M., F.C.S. and C.A.I.I.B.
Sri V.C Nannapaneni – Managing director of Natco Pharma, V.C. Nannapaneni has over 42 years of experience in the Pharmaceutical Industry. He has worked in the US for more than a decade in various Pharmaceutical companies. He holds Bachelors and Masters Degree in Pharmacy from Andhra University , Visakhapatnam, India in addition to this he also holds a Masters degree in Pharmaceutical Administration from the Brooklyn College of Pharmacy, US. Currently.
Sri Rajeev Nannapaneni –Director and Chief Executive Officer, Rajeev Nannapaneni has worked at Merill Lynch and Natco Systems LL.C in USA. He joined the Company in the year 2000. He has got wide experience and exposure in general management, new business / new product development in India and for International markets. He holds B.A degree in Quantitative Economics and also B.A in History from Tufts University, Boston, USA.
Business segments
Oncology
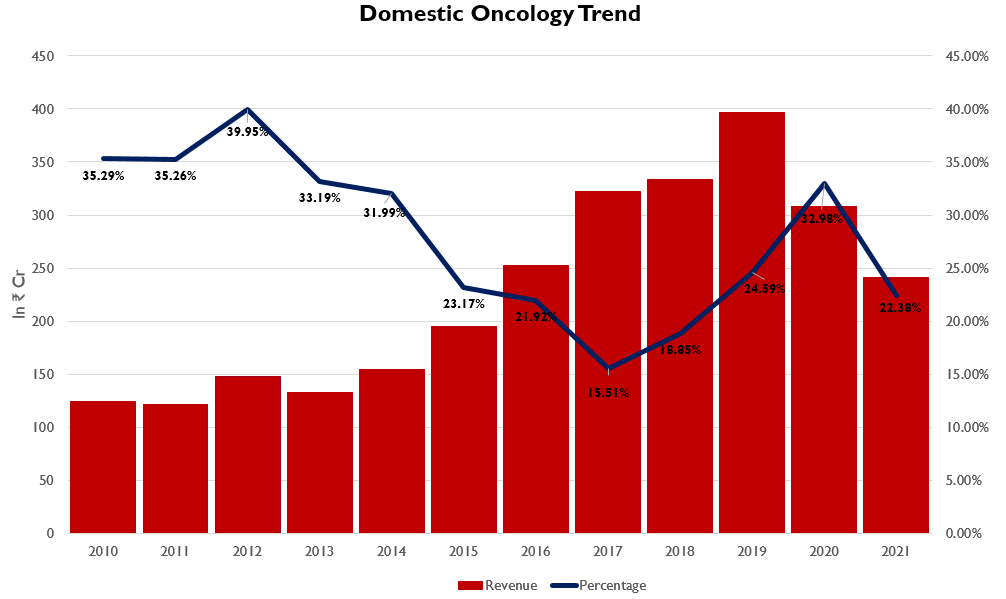
- Since 2010, Oncology has been the most stable business for Natco. It has provided a solid foundation which has enabled Natco to exploit opportunities in other segments by leveraging the business from Oncology.
- As of FY2021, Natco has 38 products under Oncology, out of which 16 are under Hematology, which is the treatment of cancer cells in the blood and 22 are under Solid Tumors.
- In FY20 the segment has seen consolidation in revenues due to the Covid disruption which saw reduced visits from cancer patients. The segment also witnessed price reductions due to Government regulations.
The top 6 products which contribute to more than ₹ 10 cr+ which are
- Veenat
- Lenalid
- Carfilnat
- Erlonat
- Geftinat
- Sorafenat
Cardio and Diabetes
- Natco had launched the C & D division as a result of increasing competition in the Oncology domestic market.
- During the year FY20, Natco launched 5 products under the C&D segment.
- This business segment is still very under utilized. The company expects the division to be the growth driver in the years to come.
Specialty
- Specialty segment includes Gastroenterology, Virology, Orthopedics. Gastroenterology which includes Hep C & B drugs. Hepatitis portfolio accounts for 90% of the specialty portfolio.
- In 2015, the Hepatitis portfolio turned out to be a blockbuster drug contributing to as much as approximately 30% to revenues in 2016-17 period alone. The drug was complex. Natco sold the Hepatitis portfolio as a combination drug. A combination drug has two or more APIs mixed together to form the final drug. For instance the combination of Sofusbivir and Ledipasvir was sold under the brand name Hepcinat LP and Sofosbuvir and Velpatasvir was sold under the brand name Velpanat.
- In recent years the Hepatitis portfolio has seen extreme consolidation as a result of increased competition and a reduction in market size.Export revenues from the Hepatitis portfolio are nominal. The segment is dominant in the domestic region.
Agro Chemicals
- The management believes that since chemistry is a strong subject of Natco, it would be sensible to enter the Agro Chemical sector.
- In the international front, Natco is going to enable partnerships. On the contrary, Natco plans to be at the front end in the domestic region.
- Natco has given guidance for this segment. It believes the sector could contribute to around 10-15% of the business in the future.
- The product mix is going to be composed of complex generics. As on FY20, the company had a capex of 100cr in Agro Chemicals.
- The Crop Health Sciences (CHS) division is established under Agro Chemicals. On the Crop Health Sciences front, NATCO launched the first indigenously manufactured pheromone-based product in India for controlling Pink Bollworm (PBW) which affects the cotton crop. They have a series of products in the pipeline, such as Chlorantraniliprole.
Geographical revenue distribution
Business in the United States
In the past, the company has taken advantage of the US pharmaceutical market. There have been products that turned out to be a blockbuster for them.
The company had launched a product called Oseltamivir. The product was launched in partnership with Alvogen. It was under a patent back then under the brand name, Tamiflu whose innovator was Gilead Sciences and was subsequently challenged by Natco Pharma under para IV. The product bagged significant revenues for Natco in the period 2017 and 2018. In 2017 Revenues of ₹704 crores were booked by Natco in FY2017. In the subsequent years, Oseltamivir market not only saw a lot of competition entering it but also the absence of flu season which was present back when Natco had just launched the product.
Fortunately for Natco, In 2018, Natco received the approval for a drug,Glatiramer acetate, which was sold under the brand name Copaxone by Teva pharmaceuticals. Natco had filed under para IV. As the run rate of Oseltamivir slowed down, the revenues from Glatiramer acetate picked up. The company was trying to get the approval from the FDA for almost 7-8 years. It was sold in a partnership with Mylan. However in 2019, the sales from Copaxone had slowed down as a result of increased competition before stabilizing at some level in FY2020.
The company is betting on a drug, Lenalidomide, which is sold under the brand name Revlimid by the innovator Celgene. Natco has entered into a settlement with the innovator company to sell a limited amount of the drug in the States. Natco has partnered with Arrow International for marketing of the drug. Apart from Natco, Dr Reddy’s have entered into a settlement with the innovator company to sell limited quantities of the drug in the US.
The management views the United States’ pharmaceutical market as highly competitive for commodity generics. The company believes in filing 6-8 products in a year consisting of complex generics.It aims at reducing R&D expenditure in the US and increasing R&D expense in the Indian market and ROW markets of Brazil and Canada.
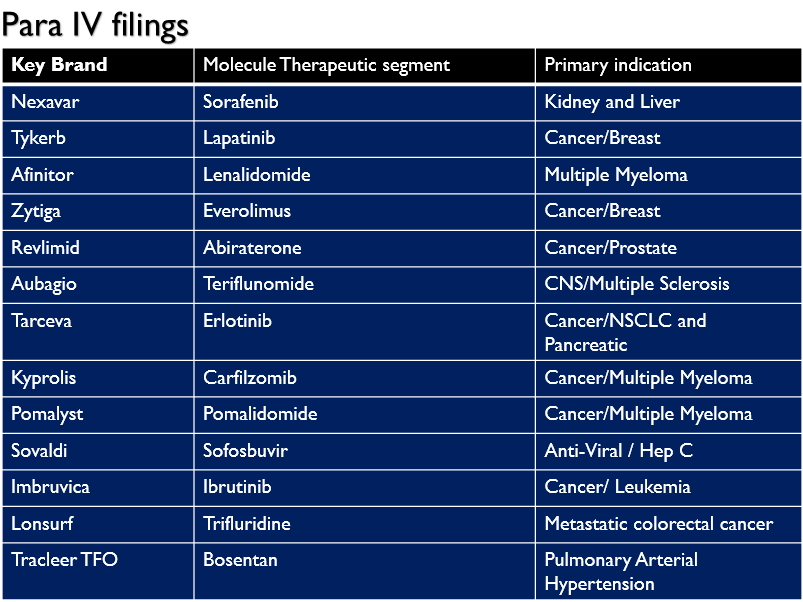
Currently, Natco has 25 commercial projects and 19 para IV filings in the United States.
Para IV filings :
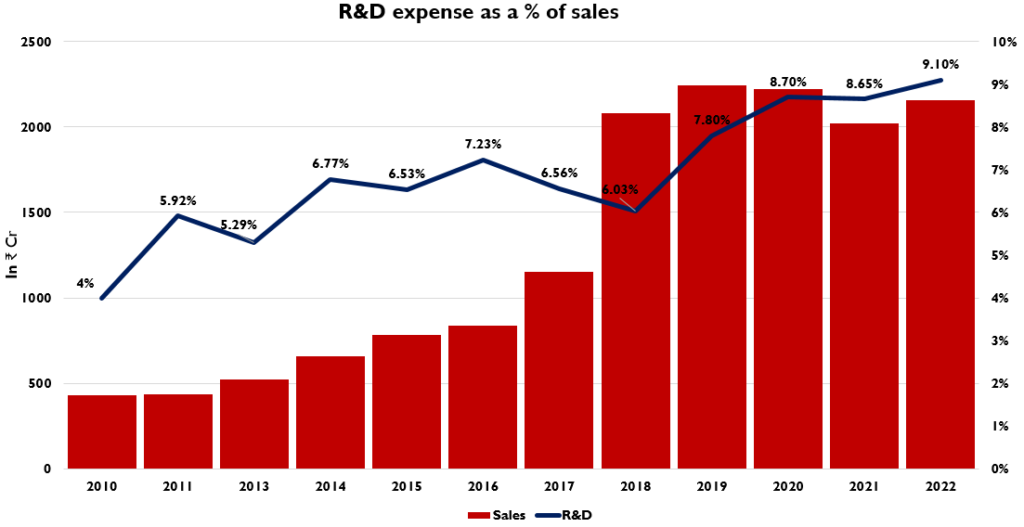
R&D expenditure
The company has an R&D team that focuses on research and development of complex generics. Its capabilities include multi-step synthesis, semi-synthetic fusion technologies, production of high-potency APIs and peptides. NATCO made initial footprints in the agrochem segment towards manufacturing of niche molecules towards pest management as a result of increased R&D over the past 2 years.
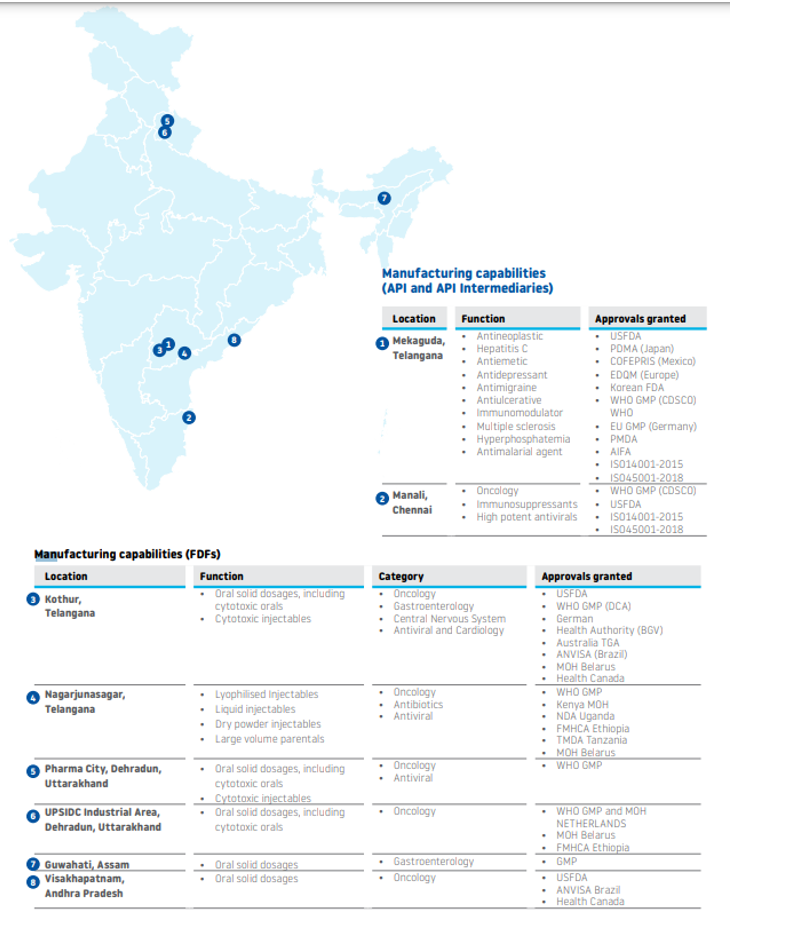
Manufacturing Plants
New ventures
- Company is looking to diversify its existing therapeutic segments in the international markets of Brazil,Canada and the like. Natco aims at expanding the Hepatitis portfolio in semi-regulated markets like Brazil, Philippines. Company is eyeing heavily on Brazil. They have a subsidiary to help them market their products and have hired a new local partner to help penetrate the Brazilian market. Company is also working towards expanding in the Canadian pharmaceutical market through its wholly owned subsidiary.
- Mr Rajeev Nannapaneni believes that players will only be able to expand their portfolio in India successfully if
- A business goes after patented products
- A company comes up with a complex unique generic
Natco plans to apply this strategy when launching products in the Indian market
- The Agro chemicals segment has been launched to not only diversify their portfolio but also to maintain their presence in the Indian and the US market to some extent.
- Management has decided to slow down their pace in the US market by filing for complex generics.