Those who follow the chemical and pharma sector often hear the terms CRO, CDMO and CRAMS. It is the direction that most chemical and pharma companies are moving in. During the race to develop a vaccine for the COVID pandemic, we often heard terms like so and so vaccine is Phase 2 or 3 of clinical trials but didn’t really understand what that meant. We are about to break down all the complex terminology around this subject and explain what these terms mean and what actually is the CRAMS industry in very simple language.
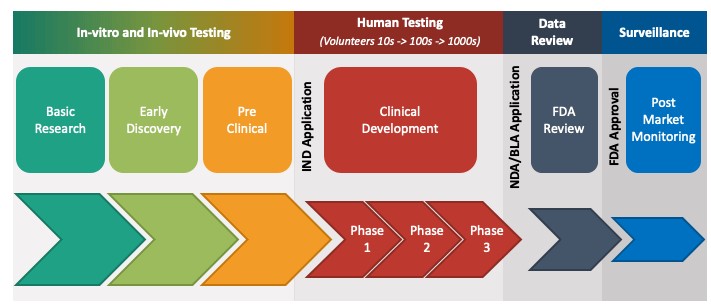
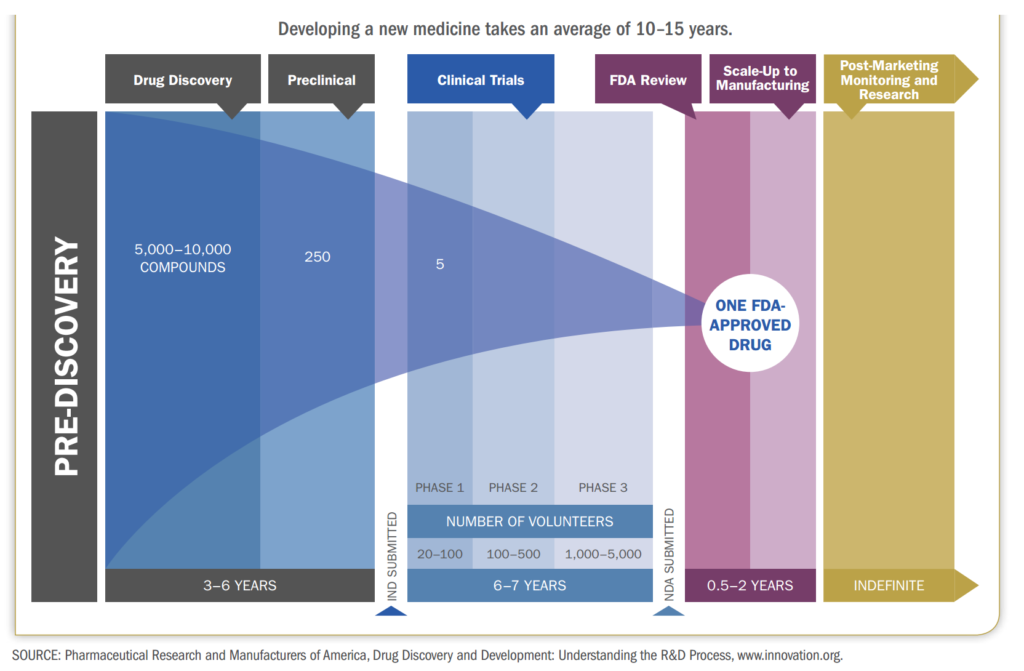
The drug development process is a very long and complex process which usually takes 10-15 years and costs hundreds of millions and a lot of times billions of dollars. This is the US FDA’s process that most new and innovative drugs have to go through before they can be sold to the public. A drug usually goes through three big phases on its way to commercialization – the discovery of the drug, the testing of the drug and manufacturing of the drug. Once we have understood this process, it will be very easy for us to understand where CROs and CDMOs come in, what is their importance and what benefits they bring to the overall value chain.
Drug Discovery
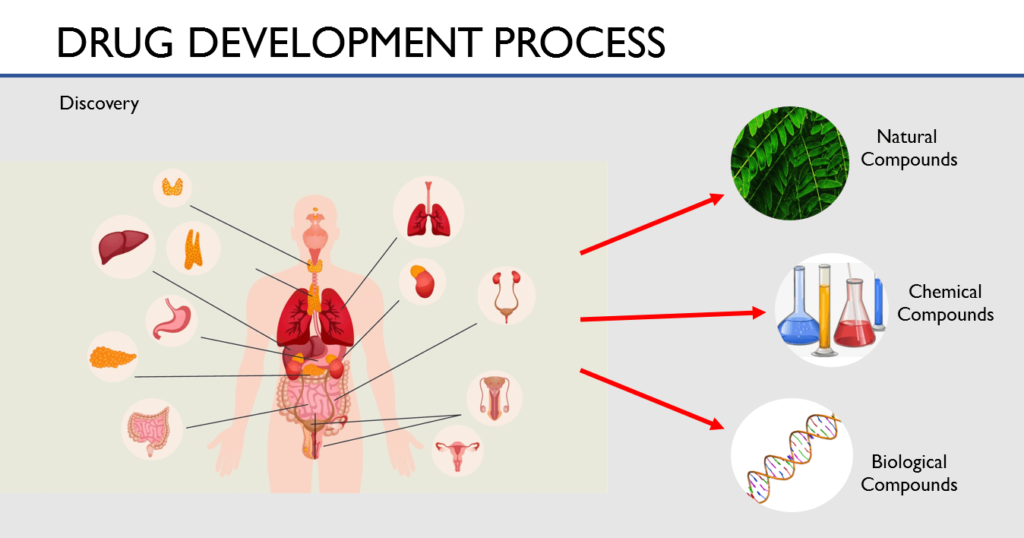
The process of drug discovery starts with the research teams who work to analyze diseases and what causes them. They analyze what part of the body a disease affects and what reaction the body has to these diseases. Once all the causes for the disease have been identified, they investigate on how to act on these causes. What would stop this disease from getting worse or even reverse their course. For this, they examine thousands of compounds like natural compounds, synthetic compounds and even bio-engineered compounds.
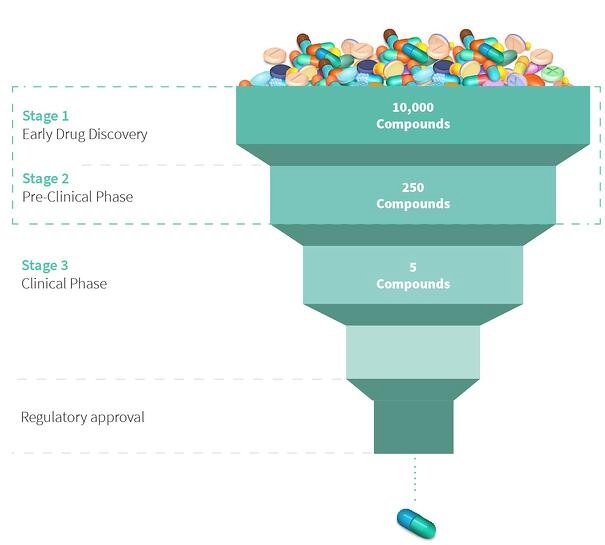
The research teams test tens of thousands of compounds on cell cultures (in-vitro testing) and in animals (in-vivo testing). This process is called the Pre-Clinical Testing phase. This helps them analyze what effect they could have on the human body. And through a process of elimination, they narrow down thousands of compounds to just a few compounds to make the drug or the medicine out of.
This is a very long and difficult process because researchers have to collect vast amounts of data on the possible side effects these drugs can have on the human body. This process usually takes about 5-6 years. Once they have narrowed down the compounds and collected all the necessary data, the researchers will file an IND – Investigational New Drug Application. Once the IND has been approved, they can now test the drug on human volunteers.
Clinical Trials
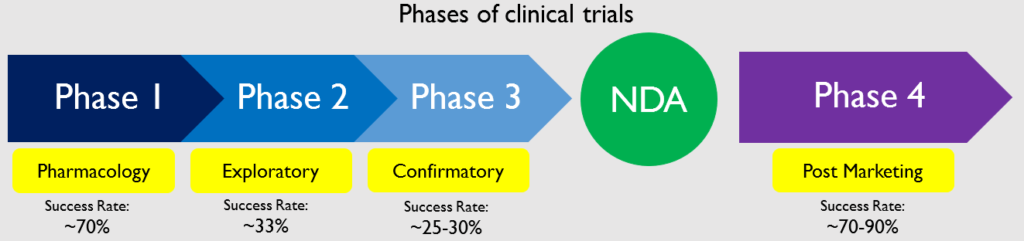
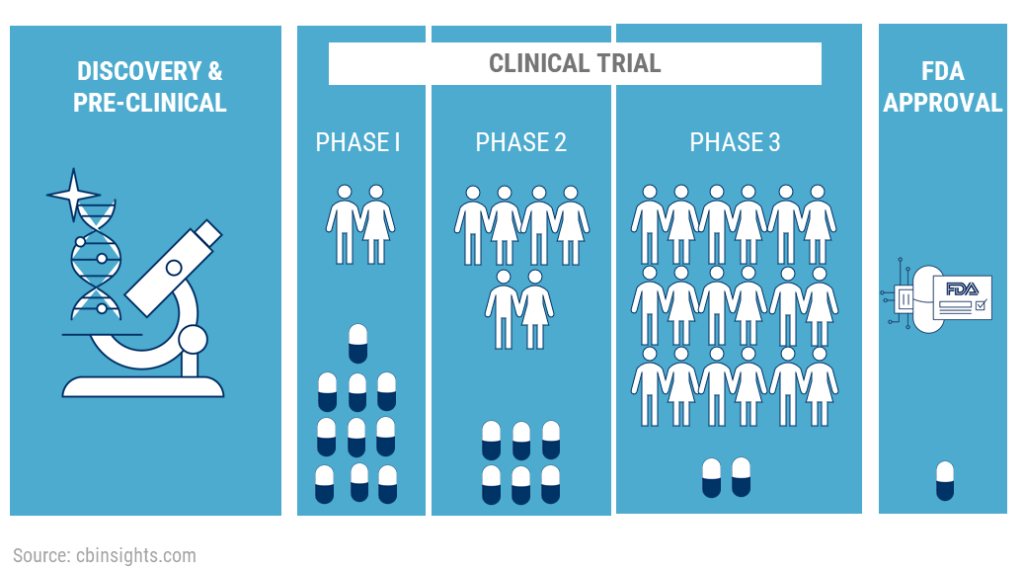
The clinical trials start after the IND is approved and is undertaken in 3 phases. Phase 1 is called pharmacology phase. The aim of the phase 1 study is to understand how the body reacts to the drug and what if there are any side effects. About 70% of the drugs in phase 1 move on to the next phase Phase 2 is called the exploratory phase. The aim of the phase 2 study is to study the safety and effectiveness of the drug. About 33% of the drugs in phase 2 move on to phase 3. Phase 3 is known as the confirmatory phase as it confirms the safety and efficacy of the drug on a wide range of people. Only 25-30% of the drugs make it past Phase 3. Phase 4 clinical trials are called post marketing study or surveillance as it happens after the drug has been released to the public. 70-90% of the drugs in this phase succeed in staying in the market.
Phase 0 Trials
Before the Phase 1 trials, researchers have to conduct a small study called human microdosing study or the Phase 0 trials. The researchers give a single sub-therapeutic dose to a very small group of people – usually 10-15. A sub-therapeutic dose is a very small dose that will not have any therapeutic effect on the body. It is done to make sure that the compound, which has never been exposed to humans, is not toxic and is safe for ingestion. If the drug acts very differently than what the researchers theorized, they might have to do some additional preclinical studies.
Phase 1
In the Phase 1 trials, researchers try to figure out what is the highest dose a human can take without any serious side effects. Researchers will also try to figure out what is the best way to deliver the drug to the human body. For example, orally- which is in the form of a tablet or capsule, through an injection, a nasal spray, etc. The study is usually conducted on a small group of healthy volunteers, usually 20-100. These volunteers have to be disease free – they cannot be suffering from any kind of condition as the researchers do not yet know how the drug will react in the body of a person suffering from certain conditions. The study usually lasts for about 6 months.
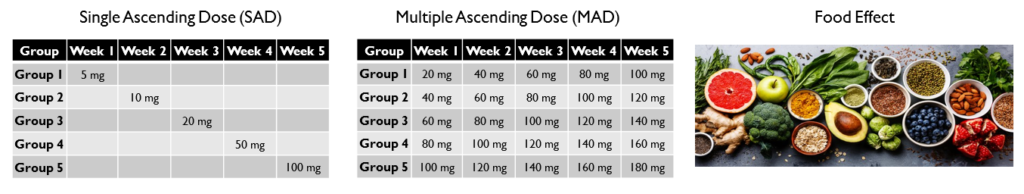
There are 3 main types of studies conducted in Phase 1 trials: Single Ascending Dose (SAD), Multiple Ascending Dose (MAD) and Food Effect.
- Single Ascending Dose (SAD): In a single ascending dose, volunteers are given a single dose of the drug and are observed for a period of time. If the drug performs as intended, the next group of volunteers are given a higher dose of the same drug. The dosage keeps increasing until the side effects of the drug become apparent. This is called the maximum tolerated dose. This study is performed to understand what dosage is ideal and what dosage will cause serious side effects.
- Multiple Ascending Dose (MAD): In multiple ascending dose, different groups of volunteers are given multiple increasing doses of the drug. This study is performed to understand how taking multiple doses of the drug over a period of time affects the body. The difference between SAD and MAD is that in SAD, each group receives only one dose of the drug whereas in MAD, each group receives multiple doses that increase with time.
- Food Effect: The researchers also conduct tests to study how food intake affects the absorption of the drug in the body. To study this, participants are given two identical doses – one while fasting and one after a meal. This is done to understand when the drug should be administered.
Phase 2
In Phase 2 of the clinical trials, the drug is now going to be tested on people actually suffering from the disease or the condition. The researchers have determined that the drug is safe for consumption and are now looking to test its efficacy – which means that they are going to see if it is actually helpful in treating the disease. This study is conducted on about 50-300 patients. They are given the same dose that was determined to be safe in phase 1. 
Phase 2 is usually conducted in 2 parts called Phase 2A and Phase 2B. In phase 2A researchers determine the ideal dose needed. And in Phase 2B they determine how effective the drug is at curing the disease at that dose. While phase 2 involves more participants than earlier phases, it’s still not large enough to demonstrate the overall safety of a medication. However, the data collected during this phase helps researchers come up with methods for conducting phase 3.
Phase 3
Phase 3 trials are the most important and provide the researchers with long term safety data. The Phase 3 trials are randomized and double-blind. Randomized means that patients are divided into 2 groups at random. 
Filing NDA
Once all these trials have been completed, the company compiles all the data from the years of preclinical and clinical studies into a document which is filed with the US FDA. This document is called an NDA which stands for New Drug Application. This is the final authorization that is needed for the company to be able to market and sell this drug.
According to the US FDA’s website, the documentation required in an NDA is supposed to tell the drug’s whole story, including what happened during the clinical tests, what the ingredients of the drug are, the results of the animal studies, how the drug behaves in the body, and how it is manufactured, processed and packaged.
What the FDA is specifically looking for is if the drug is more effective in curing the condition than the drugs that are already on the market. They have to weigh the benefits that the drug provides against the side effects and only approve those drugs in which the benefit outweighs the cost.
Phase 4
Even though the drug has been approved for sale, the testing of the drug is not over. Phase 4 testing is called pharmacovigilance. In Phase 4, the long term effects of the drug are studied after it is released to the public. When the drug is marketed globally, it is taken by a diverse group of people from different regions who also suffer from various other diseases. The effects of the drug are monitored and if the drug has any adverse effects, it is recalled. 70-90% of the drugs that make it to phase 4 succeed in staying on the market.
Manufacturing of the drug
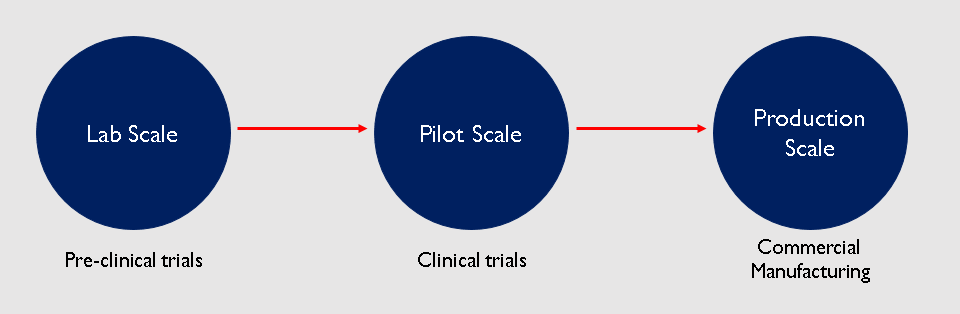
Now that the drug has been approved for sale, the pharma company has to scale up production to manufacture it on a commercial level. During pre-clinical trials, very small quantities of the drug were manufactured in the lab for testing. So the drug was produced at a lab scale. Then if the drug made it to clinical trials, the production had to be scaled up to manufacture the drug for a couple thousand people. This is known as pilot scale. And if the drug actually receives approval, it has to be scaled up to make millions of doses for sale to the public.
Role of CROs and CDMOs
Now that we understand the process of bringing innovative drugs to market, we can easily understand the role of CROs and CDMOs.
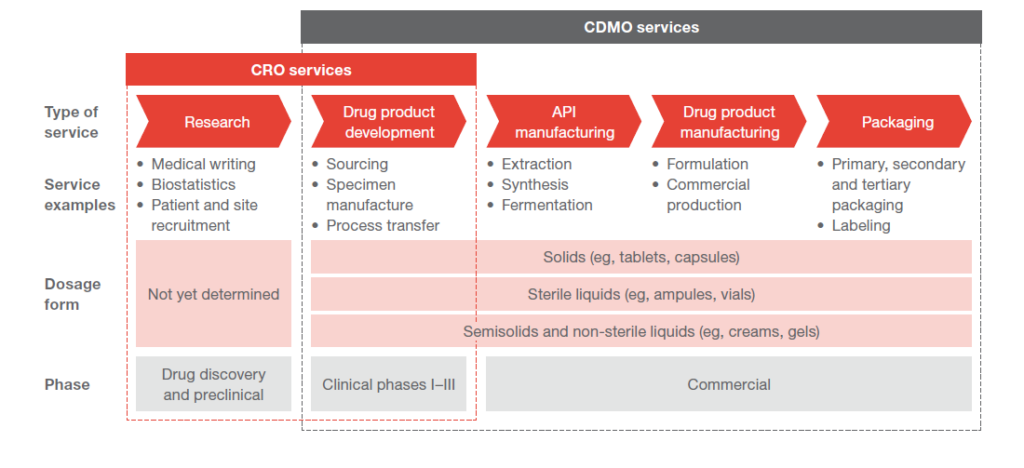
A CDMO is not involved with the preclinical phase. They come in after the drug receives clearance to be tested on humans. They help in scaling up the drug from lab scale to pilot scale, and if approved, they help scale the drug to production scale. They assist the pharma company in developing the API for the drug and the formulation. They also manufacture and package the drug for sale in the market. So they are more like partners for the pharma companies because they help bring the drug to market right from the trials stage.
Contract manufacturing firms are not concerned with the development of the drug. Rather, they come in when the drug is approved and needs to be mass manufactured.
There are also fully integrated players in the market who provide all of the above services. From target discovery to manufacturing of the final drug This is called CRAMS – Contract Research And Manufacturing Services. So CRAMS is basically a CRO and a CDMO. They are treated as partners by the pharma companies who often co invest in manufacturing facilities.
CRAMS is a mutually beneficial relationship for the pharma company and the CDMO. Think about this. If you are a pharma company and you are developing a new drug, you will have to invest in capacities before a drug has been approved to reduce time to market. If the drug fails in Phase 3 trials, then that investment has gone to waste. So by hiring a CDMO, a pharma company can be asset light. Besides, CDMOs also have expertise in manufacturing the drug at a large scale which the pharma company may not have. The Pharma company may also choose to manufacture the drug themselves and have the CDMO as a backup in case they face issues in the future with their own manufacturing facilities. And with the manufacturing side of things taken care of, the pharma company can focus on innovation and not worry about the complexity that goes into manufacturing the drug.
The manufacturer on the other hand receives stable and predictable cash flows. CDMOs are paid to take the drug through clinical trials. If the drug fails, they still get paid. But if the drug is approved, they get to manufacture the drug at a commercial scale. Indian companies do not come up with innovative drugs which is a very high margin business. Indian manufacturers are more focused on generic drugs. This is a way for Indian players to get a piece of the high margin business of patented drugs. The innovators also provide the CDMO with the technology to manufacture these drugs which they may not have had access to in any other scenario.